 A water softener removes minerals from your water using “ionic exchange”. An ion is an atom that has either a positive (+) or negative (-) charge. And if you remember from your high school chemistry class, you know that opposites attract!
A water softener removes minerals from your water using “ionic exchange”. An ion is an atom that has either a positive (+) or negative (-) charge. And if you remember from your high school chemistry class, you know that opposites attract!
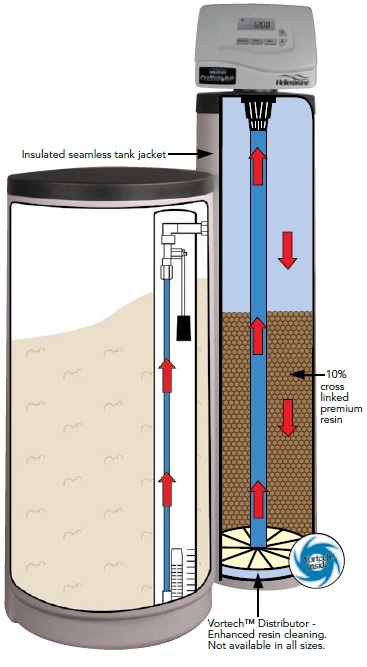
Iron, manganese, and hard water, are all minerals with a positive charge (+). So it makes sense that to remove them, you would need something with a negative charge, right? The Softener contains special polymer beads, called resin, with a (-) charge. So as the positively charged minerals pass through a bed of negatively charged resin, the minerals are attracted to and “stick” to the bead.
But there is an inherent problem. What happens when the beads are full? How do you “clean” the beads and push the minerals off? The secret answer is…..SALT!
Salt is really sodium (+) chloride (-). When your Softener goes through a cycle, salty water is drawn from the salt tank and into the tall tank which holds the resin beads. The salty water is strong enough to push the minerals off the beads, and “clean” the beads. All of the undesirable minerals like iron, manganese and hardness go to drain. What is left behind is the sodium (+) now clinging to the resin bead (-).
 Think of this as a continuous cycle. As you use water in your house, the untreated water that contains the dissolved minerals will replace or “exchange places” with the sodium, until the bead is full of minerals. When the bead is full, another cleaning cycle is needed.
Think of this as a continuous cycle. As you use water in your house, the untreated water that contains the dissolved minerals will replace or “exchange places” with the sodium, until the bead is full of minerals. When the bead is full, another cleaning cycle is needed.
So, the answer to “Why salt?” is that it meets two important criteria:
1) It is really inexpensive and non-toxic
2) It easily allows for the ionic exchange to occur and gives you delicious, treated water.
